Abstract
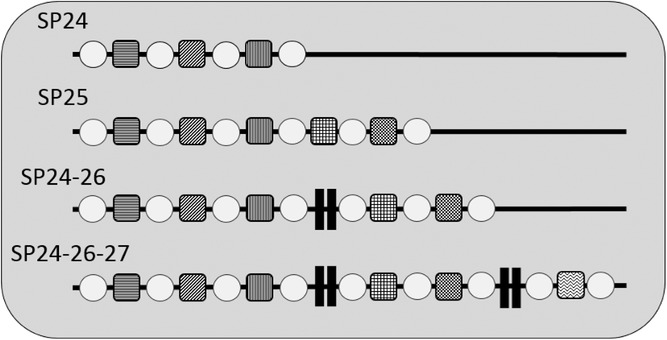
The foodborne pathogen Listeria monocytogenes can persist in food-associated environments for long periods. To identify persistent strains, the subtyping method pulsed-field gel electrophoresis (PFGE) is being replaced by whole genome sequence (WGS)-based subtyping. It was hypothesized that analyzing specific mobile genetic elements, CRISPR (Clustered Regularly Interspaced Short Palindromic Short Repeat) spacer arrays, extracted from WGS data, could differentiate persistent and sporadic isolates within WGS-based clades. To test this hypothesis, 175 L. monocytogenes isolates, previously recovered from retail delis, were analyzed for CRISPR spacers using CRISPRFinder. These isolates represent 23 phylogenetic clades defined by WGS-based single nucleotide polymorphisms and closely related sporadic isolates. In 174/175 (99.4%) of isolates, at least one array with one spacer was identified. Numbers of spacers in a single array ranged from 1 to 28 spacers. Isolates were grouped into 13 spacer patterns (SPs) based on observed variability in the presence or absence of whole spacers. SP variation was consistent with WGS-based clades forming patterns of (i) one SP to one clade, (ii) one SP across many clades, (iii) many SPs within one clade, and (iv) many SPs across many clades. Unfortunately, SPs did not appear to differentiate persistent from sporadic isolates within any WGS-based clade. Overall, these data show that (i) CRISPR arrays are common in WGS data for these food-associated L. monocytogenes and (ii) CRISPR subtyping cannot improve the identification of persistent or sporadic isolates from retail delis. Practical Application: CRISPR spacer arrays are present in L. monocytogenes isolates and CRISPR spacer patterns are consistent with previous subtyping methods. These mobile genetic artifacts cannot improve the differentiation between persistent and sporadic L. monocytogenes isolates, used in this study. While CRISPR-based subtyping has been useful for other pathogens, it is not useful in understanding persistence in L. monocytogenes. Thus, the food safety community might be able to use CRISPRs in other areas, but CRISPRs do not seem likely to improve the differentiation of persistence in L. monocytogenes isolates from retail delis.